
T-OPAL® Thermotropic Casting-Resin Panes
Thermo-optically variable polymeric material with adaptive light transmission
Thermotropic Casting-Resin Panes
Thermo-optically variable polymeric material with adaptive light transmission
In the picture below we see an room, partially glazed with T-OPAL® casting-resin panes.

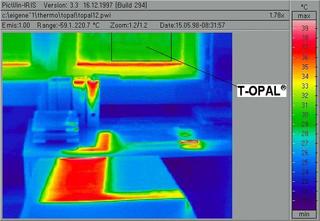
From the same position, the suceeding picture shows a thermographic photo The temperature-controlled opacity of the T-OPAL® system at high outside temperatures reduces the solar radiation in the interior. This effect is proved by the reduced surface temperatures of the furniture behind the panes (blue area in the thermogram), whereas normal insulating glass leads to a high temperature of the furniture surfaces, an effect that is obvious in the left part of the photo (red area in the thermogram).
How it works: The T-OPAL® system that uses a technology called thermally reversible light scattering - the glass is frosted (translucent) when it’s cold, but turns completely transparent when heated. The material is stable to about 85 degrees C.
1.At activation temperature (adjustable between about -10 degrees C. to 50 degrees C.) glazing becomes transparent - this takes roughly 2-3 minutes for a 28 x 18 piece.
2.When material reaches ambient temperature it becomes opaque. It can take from 20 minutes to 2 hours to complete the transformation depending on the temperature the glass was raised to, the ambient temperature, and the formula used to make the T-OPAL® material.
Specifically, at Critical temperature (HOT) the material goes through a physical electronic transformation in specific transition metal oxides and related compounds which transfers it from a semi-conducting state to a metallic state. the incident light is strongly scattered, with most of it being diffused.
Conversely, at the Ambient temperature the material goes through a physical electronic transformation which transfers it from a metallic state to a semi-conducting state causing multiple absorption of light - only a small fraction is transmitted.
Perhaps this passive technology can be transformed to create private and semi-private work clusters in learning environments? --Mikael Powell
No comments:
Post a Comment